3.3 Palaeo CO2 and Natural Changes in the Carbon Cycle
3.3.1 Geological History of Atmospheric CO2
Atmospheric CO2 concentration has varied on all time-scales during
the Earth’s history (Figure 3.2). There is
evidence for very high CO2 concentrations (>3,000 ppm) between
600 and 400 Myr BP and between 200 and 150 Myr BP (Figure
3.2f). On long time-scales, atmospheric CO2 content is determined
by the balance among geochemical processes including organic carbon burial in
sediments, silicate rock weathering, and vulcanism (Berner, 1993, 1997). In
particular, terrestrial vegetation has enhanced the rate of silicate weathering,
which consumes CO2 while releasing base cations that end up in the
ocean. Subsequent deep-sea burial of Ca and Mg (as carbonates, for example CaCO3)
in the shells of marine organisms removes CO2. The net effect of
slight imbalances in the carbon cycle over tens to hundreds of millions of years
has been to reduce atmospheric CO2. The rates of these processes
are extremely slow, hence they are of limited relevance to the atmospheric CO2
response to emissions over the next hundred years.
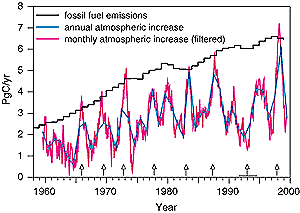
Figure 3.3: Fossil fuel emissions and the rate of increase of CO2
concentration in the atmosphere. The annual atmospheric increase is the
measured increase during a calendar year. The monthly atmospheric increases
have been filtered to remove the seasonal cycle. Vertical arrows denote
El Niño events. A horizontal line defines the extended El Niño
of 1991 to 1994. Atmospheric data are from Keeling and Whorf (2000), fossil
fuel emissions data are from Marland et al. (2000) and British Petroleum
(2000), see explanations in text. |
It is pertinent, however, that photosynthesis evolved at
a time when O2 concentrations were far less than at present. O2
has accumulated in the atmosphere over geological time because photosynthesis
results in the burial of reduced chemical species: pyrite (FeS2)
derived from sulphur-reducing bacteria, and organic carbon. This accumulation
has consequences for terrestrial and marine ecosystems today. Primary production
is carbon limited in terrestrial ecosystems in part because of (geologically
speaking) low CO2 concentrations, and in part because Rubisco (the
enzyme that fixes CO2 in all plants) also has an affinity for O2
that reduces its efficiency in photosynthesis (see Section
3.2.2.4). Primary production is iron limited in some marine ecosystems mainly
because of the extreme insolubility of Fe(III), the predominant form of iron
in the present, O2-rich environment. These difficulties faced by
contemporary organisms represent a legacy of earlier evolution under very different
biogeochemical conditions.
In more recent times, atmospheric CO2 concentration continued to
fall after about 60 Myr BP and there is geochemical evidence that concentrations
were <300 ppm by about 20 Myr BP (Pagani et al., 1999a; Pearson and Palmer,
1999, 2000; Figure 3.2e). Low CO2 concentrations may have been the
stimulus that favoured the evolution of C4 plants, which increased
greatly in abundance between 7 and 5 Myr BP (Cerling et al., 1993, 1997; Pagani
et al., 1999b). Although contemporary CO2 concentrations were exceeded
during earlier geological epochs, they are likely higher now than at any time
during the past 20 million years.
3.3.2 Variations in Atmospheric CO2 during Glacial/inter-glacial
Cycles
The purity of Antarctic ice allows the CO2 concentration in trapped
air bubbles to be accurately measured (Tschumi and Stauffer, 2000). The CO2
record from the Vostok ice core is the best available for the glacial/inter-glacial
time-scale and covers the past four glacial/inter-glacial cycles (420 kyr) with
a resolution of 1 to 2 kyr (Petit et al., 1999; Fischer et al., 1999). The general
pattern is clear (Figure 3.2d): atmospheric CO2
has been low (but 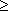 180 ppm)
during glacial periods, and higher (but
180 ppm)
during glacial periods, and higher (but 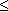 300
ppm) during interglacials. Natural processes during the glacial-interglacial
cycles have maintained CO2 concentrations within these bounds, despite
considerable variability on multi-millenial time-scales. The present CO2
concentration is higher than at any time during the 420 kyr period covered by
the Vostok record.
300
ppm) during interglacials. Natural processes during the glacial-interglacial
cycles have maintained CO2 concentrations within these bounds, despite
considerable variability on multi-millenial time-scales. The present CO2
concentration is higher than at any time during the 420 kyr period covered by
the Vostok record.
The terrestrial biosphere stores 300 to 700 Pg more carbon during interglacial
periods than during glacial periods, based on a widely accepted interpretation
of the 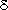 13C record
in deep-sea sediments (Shackleton, 1977; Bird et al., 1994; Crowley, 1995).
Terrestrial modelling studies (e.g., Friedlingstein et al., 1995b; Peng et al.,
1998) have reached the same conclusion. Thus, the terrestrial biosphere does
not cause the difference in atmospheric CO2 between glacial and interglacial
periods. The cause must lie in the ocean, and indeed the amount of atmospheric
change to be accounted for must be augmented to account for a fraction of the
carbon transferred between the land and ocean. The mechanism remains controversial
(see Box 3.4). In part this is because a variety
of processes that could be effective in altering CO2 levels on a
century time-scale can be largely cancelled on multi-millenial time-scales by
changes in CaCO3 sedimentation or dissolution, as discussed in Section
3.2.3.1.
13C record
in deep-sea sediments (Shackleton, 1977; Bird et al., 1994; Crowley, 1995).
Terrestrial modelling studies (e.g., Friedlingstein et al., 1995b; Peng et al.,
1998) have reached the same conclusion. Thus, the terrestrial biosphere does
not cause the difference in atmospheric CO2 between glacial and interglacial
periods. The cause must lie in the ocean, and indeed the amount of atmospheric
change to be accounted for must be augmented to account for a fraction of the
carbon transferred between the land and ocean. The mechanism remains controversial
(see Box 3.4). In part this is because a variety
of processes that could be effective in altering CO2 levels on a
century time-scale can be largely cancelled on multi-millenial time-scales by
changes in CaCO3 sedimentation or dissolution, as discussed in Section
3.2.3.1.
|
Box 3.5: The use of O2 measurements to
assess the fate of fossil fuel CO2.
The amount of CO2 that remains in the atmosphere each year
has been consistently less than the amount emitted by fossil fuel burning.
This is because some CO2 dissolves and mixes in the ocean,
and some is taken up by the land. These two modes of uptake have different
effects on the concentration of O2 in the atmosphere. Fossil
fuel burning consumes O2 and causes a decline in atmospheric
O2 concentration (Figure 3.4).
Dissolution of CO2 in the ocean has no effect on atmospheric
O2. Terrestrial uptake of CO2, by contrast, implies
that photosynthesis (which releases O2) is exceeding respiration
and other oxidation processes, including fire (which consume O2).
Thus, net terrestrial uptake of CO2 implies a net release of
O2, in a known stochiometric ratio. This difference can be
used to partition the total CO2 uptake into land and ocean
components, as shown graphically in Figure 3.4.
Strictly speaking, the atmospheric O2 – CO2
budget method can only distinguish between net non-biological ocean uptake
and net biospheric uptake, which in principle includes both the terrestrial
and the marine biosphere. However, since biological oxygen uptake is not
expected to have changed significantly during recent decades because of
nutrient limitations in most parts of the ocean (see Section
3.2.3.2), this inferred biospheric uptake is attributed to the land.
Measurement of changes in O2 presents a technical challenge
because changes of a few ppm caused by fossil fuel burning have to be
determined against a background concentration of 209,000 ppm (about 21%).
For technical reasons, O2 is measured relative to N2,
the main constituent of the atmosphere, as a reference gas. For simplicity
this chapter refers to O2 concentrations, although strictly
it is O2 : N2 ratios that are measured. The impact
of nitrification-denitrification changes on atmospheric N2
content are assumed not to be problematic because they are small and the
inventory of N2 is very large. Increases in ocean temperatures
(Levitus et al., 2000), because of their effect on the temperature dependent
solubility, induce small outgassing fluxes of O2 and N2
(Keeling et al., 1993) that have to be taken into account (see Figure
3.4) although their magnitude is only approximately known. Impacts
on atmospheric O2 caused by changes in the ventilation of deeper,
oxygen depleted waters have been observed on interannual time-scales (Keeling
et al., 1993, Bender et al., 1996). They could also occur on longer time-scales,
e.g., through increased ocean stratification induced by ocean warming.
|
Orbital variations (Berger, 1978) are the pacemaker of climate change on multi-millenial
time-scales (Hays et al., 1976). Atmospheric CO2 is one of many Earth
system variables that show the characteristic “Milankovitch” periodicities,
and has been implicated as a key factor in locking natural climate changes to
the 100 kyr eccentricity cycle (Shackleton, 2000). Whatever the mechanisms involved,
lags of up to 2,000 to 4,000 years in the drawdown of CO2 at the
start of glacial periods suggests that the low CO2 concentrations
during glacial periods amplify the climate change but do not initiate glaciations
(Lorius and Oeschger, 1994; Fischer et al., 1999). Once established, the low
CO2 concentration is likely to have enhanced global cooling (Hewitt
and Mitchell, 1997). During the last deglaciation, rising CO2 paralleled
Southern Hemisphere warming and was ahead of Northern Hemisphere warming (Chapter
2).
During glacial periods, the atmospheric CO2 concentration does not
track the “fast” changes in climate (e.g., decade to century scale
warming events) associated with Dansgaard-Oeschger events, although there are
CO2 fluctuations of up to 20 ppm associated with the longer-lived
events (Stauffer et al., 1998; Indermühle et al., 2000) (see Chapter 2
for explanations of these terms). During the last deglaciation, atmospheric
CO2 concentration continued to increase, by about 12 ppm, through
the Younger Dryas cold reversal (12.7 to 11.6 kyr BP) seen in Northern Hemisphere
palaeoclimate records (Fischer et al., 1999; Smith et al., 1999). Palaeo-oceanographic
evidence shows that the Younger Dryas event was marked by a prolonged shut-down
of the thermohaline circulation, which is likely to have been triggered by the
release of melt water into the North Atlantic. Similar behaviour, with a slight
rise in CO2 accompanying a major Northern Hemisphere cooling and
shutdown of North Atlantic Deep Water production, has been produced in a coupled
atmosphere-ocean model (Marchal et al., 1998). The observed CO2 rise
during the Younger Dryas period was modest, suggesting that atmospheric CO2
has, under natural conditions, been well buffered against abrupt changes in
climate, including thermohaline collapse. This buffering is a direct consequence
of the large reservoir of DIC in the ocean.
