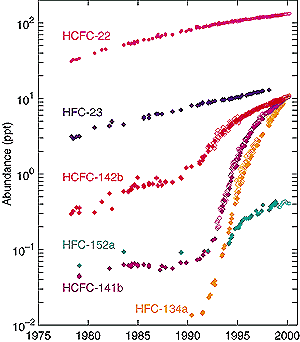
Figure 4.3: HFC-23 (blue, UEA scale), -152a (green, UEA scale), -134a (orange, NOAA scale), and HCFC-22 (magenta, SIO scale), -142b (red, NOAA scale), and -141b (purple, NOAA scale) abundances (ppt) at Cape Grim, Tasmania for the period 1978 to 1999. Different symbols are data from different measurement networks: SIO (filled circles), NOAA-CMDL (open diamonds, Montzka et al., 1994, 1996a,b, 1999), UEA (filled diamonds, Oram et al., 1995, 1996, 1998, 1999) and AGAGE (open circles, only for 1998 to 2000, all gases but HFC-23, Miller et al., 1998; Sturrock et al., 1999; Prinn et al., 2000). Southern Hemisphere values (Cape Grim) are slightly lower than global averages.